Which Best Describes a Radioactive Isotope
Deuterium is not radioactive and is the form of hydrogen found in heavy water. Three more neutrons b.

Radioactive Isotope Description Uses Examples Britannica
8 6 and 4.

. How to calculate atomic weight of a given chlorine sample when two isotopes are mixed. Three more protons c. But this was marked down as an anomaly caused by the plastic container used to carry the sample.
The latest urine test had revealed the presence of a new radioactive isotope polonium-210. The radioactive isotope is bound to a modified glucose molecule that can be taken up by cells but neither fully metabolized nor excreted. 8 6 and 6 d.
You cant live without water. Identify the choice that best completes the statement or answers the question. Radioactive elements like uranium and hafnium are trapped inside the minerals that make up these objects when they form which allows planetary scientists to tell how old they are.
The isotopic labeling of biomolecules with stable isotope-containing molecules or radioactive compounds frequently is used for detection assays mass spectrometry analysis and imaging applications. What is Mendeleev is known for. What is its chemical formula.
Because cancer cells typically get their energy from anaerobic glycolysis rather than the oxidative phosophorylation pathway they consume glucose at a higher rate and so accumulate large quantitites of radioactive glucose. A particular carbon isotope has an atomic number of 6 and an atomic mass of 14. Add the obtained values in step 1 For each isotope specified in the sample.
This chapter reviews the principle reagents and isotopes used in bioconjugate techniques including bifunctional chelating agents and radioimmunoconjugates iodination. Phosphorus-32 radioactive has _____ than phosphorus-35 normal. Most of the hydrogen in the world is protium one proton no neutrons but some deuterium and tritium occurs too.
The atomic mass of the second isotope is 3696590 and the abundance is 2422. Yin Jiang EyeEm. 6 8 and 6 b.
Hydrogen only has one isotope. Publishing the first periodic table B. 8 6 and 8 e.
6 6 and 8 c. Creating todays atomic model C. Japan plans to release into the sea more than 1 million tonnes of treated radioactive water from the destroyed Fukushima nuclear station the government said on.
The respective number of neutrons protons and electrons that this carbon isotope has is _____. Using these measurements and simulations of the physics of dust and planetesimal collisions planetary scientists and astronomers have established that the dust-to-protoplanet process. The atomic mass of the first isotope is 3496885 and the abundance is 7578.
Each column of the periodic table is A.

What Are Radioactive Isotopes Properties Of Matter Chemistry Fuseschool Youtube

How Radioactive Isotopes Are Used In Medicine Britannica
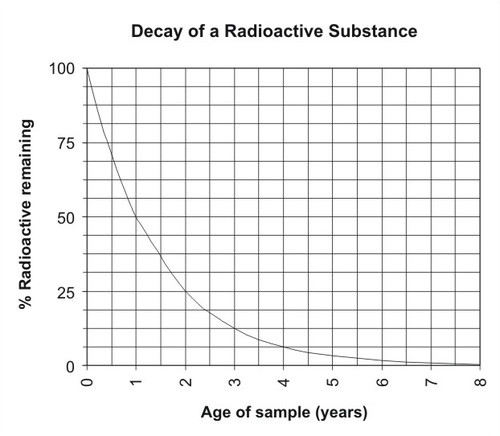
Radioactive Decay As A Measure Of Age Read Earth Science Ck 12 Foundation

Solved Which Of The Following Best Describes A Natural Chegg Com

Concept Map Activity Rock Types Concept Map Map Activities Rock Types

Radioactivity In Minerals Are Caused By The Inclusion Of Naturally Occurring Radioactive Elements In The Min Rocks And Minerals Minerals And Gemstones Minerals

Which Best Describes A Radioactive Isotope An Atom Is Unstable An Atom Is Stable There Is Only Brainly Com

Cave Of Crystals Giant Crystal Cave At Naica Mexico Crystal Cave Giant Crystal Crystals
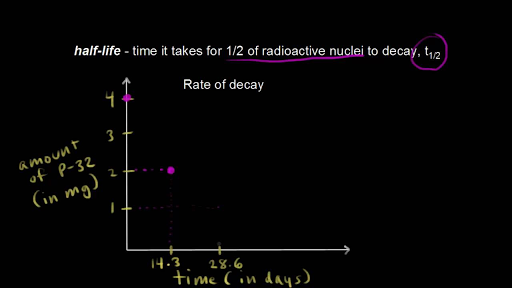
Half Life Plot Video Nuclei Khan Academy

Garnet Group The Colors And Varieties Of Garnet Garnet Minerals And Gemstones Garnet Crystal

Types Of Iridescent Gemstones Minerals Crystals And Gemstones Gemstones Chart Gemstones

Crystal Structure And Crystal Systems Crystal System Crystal Structure Crystals

17 6 Radiocarbon Dating Using Radioactivity To Measure The Age Of Fossils And Other Artifacts Chemistry Libretexts
Difference Between Stable And Unstable Isotopes Definition Properties Applications

The World S 10 Most Deadly Minerals Minerals Rock Minerals Minerals And Gemstones
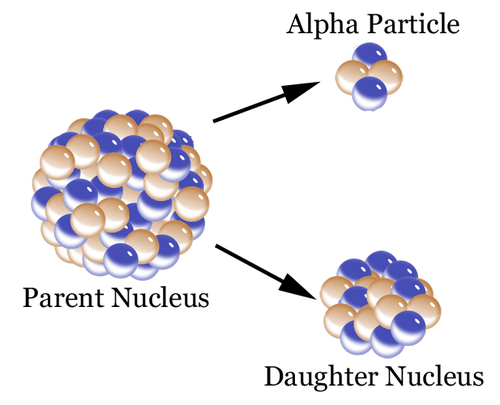
Radioactive Decay As A Measure Of Age Read Earth Science Ck 12 Foundation

Radioactive Decay Definition Radioactive Decay Law Types Of Radioactive Decay
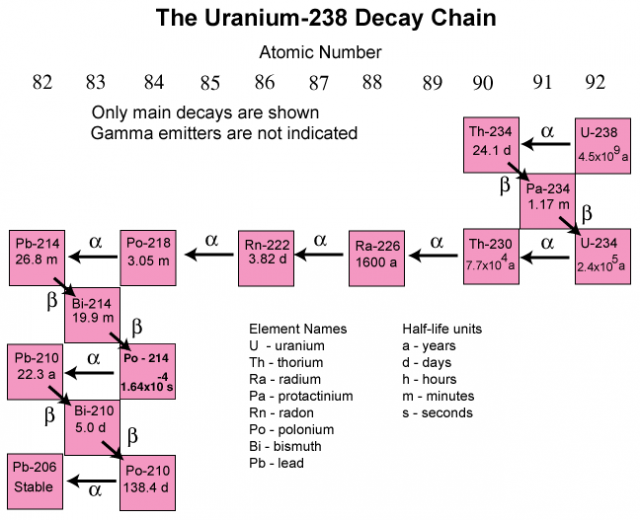
Comments
Post a Comment